Abstract
The outcome of Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) have greatly improved in tyrosine kinase inhibitors (TKIs) era and is just moving to a chemo-free era using dasatinib and blinatumomab (Foà R, N Engl J Med. 2020; Ravandi F, Blood. 2019). However, the outcome of T315I/compound-mutated Ph+ ALL patients is dismal (Cortes JE, N Engl J Med. 2013), representing an unmet need. Recently, Scherr et al reported the curative potential of venetoclax-TKIs-dexamethasone in a BCR-ABL+ mouse model (Scherr M, Leukemia. 2019).
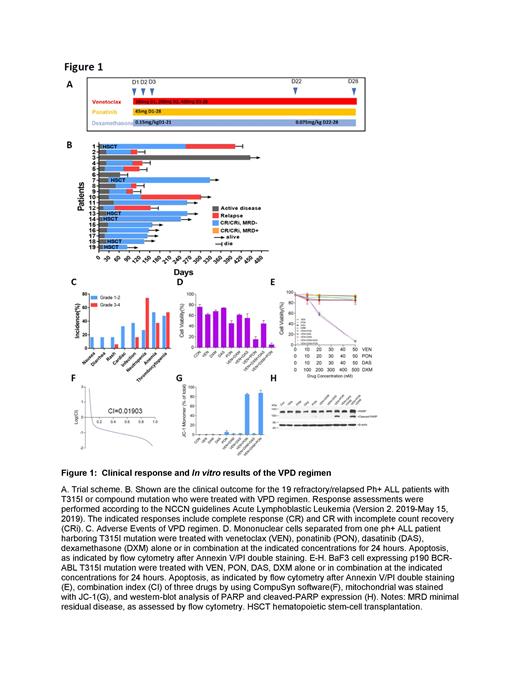
Here, we firstly reported outcome of 19 T315I/compound-mutated relapsed/refractory (R/R)Ph+ ALL patients treated with venetoclax(100mg d1, 200mg d2, 400mg d3-28), ponatinib (45mg d1-28) and dexamethasone (0.15mg/kg d1-21,0.075mg/kg d22-28)(VPD regimen) (Figure 1A) between January 2020 and May 2021. They had already received a median of 3 lines of salvage therapy.
After one cycle, 17 patients (89.5%) achieved CR/CRi [13 minimal residual disease (MRD)--negative by flow cytometry;11 major molecular remission (MMR);8 complete molecular remission (CMR)] (Figure 1B). Subsequently relapse occurred in 1/6 [allogeneic hematopoietic cell transplantation (allo-HSCT) group)] and 7/11 (VDP consolidation group). At a median follow-up of 259 days, the median EFS and OS of patients from the starting VPD treatment was 242 and 400 days. Adverse events of VPD regimen were listed in Figure 1C. Grade 3-4 neutropenia, anemia and thrombocytopenia occurred in 73.7%,36.8% and 52.6% patients. 5.3% and 16% patients have grade 3-4 rash and infection separately. No tumor lysis syndrome or death occurred. 7/19 patients were treated safety outpatient. Moreover, venetoclax had a strong synergistic effect with ponatinib and dexamethasone on inducing apoptosis of primary blast cells and BaF3 cells expressing p190 BCR/ABL with T315I-mutation in vitro, with a combination index of 0.019 when the suppressing rate is 0.05, while the effect was significant decreased when ponatinib was replaced by dasatinib (Figure 1D-F), a prominent change of mitochondrial membrane potential as well as the cleavage of PARP were also observed in triple-combination treatment group (Figure 1G-H).
For T315I/compound-mutated Ph+ ALL, VPD regimen exhibited 89.5% CR/CRi rate, with deep molecular remission (57.9% MMR), while ponatinib alone showed 41% hematologic response (Cortes JE, N Engl J Med. 2013), which supported by the preclinical data suggesting TKIs and venetoclax are highly synergistic in BCR-ABL + cells in vitro (Scherr M, Leukemia. 2019). 7/11 and 1/6 patients subsequently relapsed in continuous VPD and allo-HSCT postremission treatment group separately, suggested bridging to allo-HSCT after remission is warranted. Moreover, novel compounds such as blinatumomab showed a preliminary efficacy (Couturier MA, Leuk Lymphoma. 2021). In summary, VPD regimen provides a novel treatment for T315I/compound-mutated R/R Ph+ALL under a complete oral and chemo-free model. A clinical trial also using similar VPD regimen for treatment of R/R Ph+ ALL is ongoing now (Short NJ, Am J Hematol. 2021).
No relevant conflicts of interest to declare.